|
|
|||||||||||||||||||||||||||||||||
|
|
|||||||||||||||||||||||||||||||||
|
The latest 2008 study
detected hepatitis
contamination by PCR 8% of MUNJI injections contaminated.
|
|
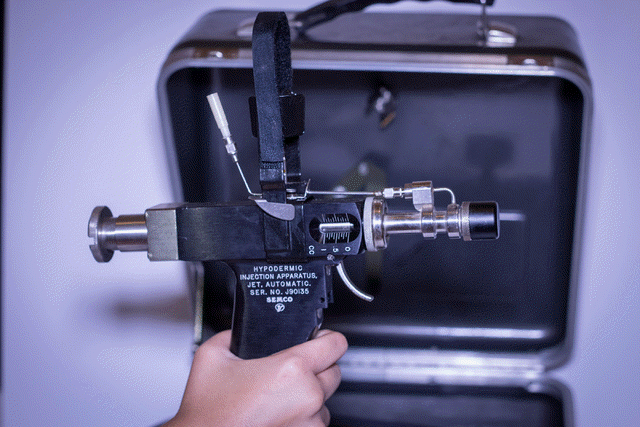
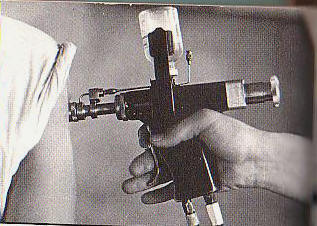
The Unintended Consequences of Vaccine Delivery Devices Used to Eradicate Smallpox: Lessons for Evaluating Future Vaccination Methods B. G. Weniger 1, T. S. Jones2, R. T. Chen31Associate Editor, Vaccine, CAPT, USPHS (ret.), Atlanta, GA, 2CAPT, USPHS (ret.), Florence, MA, 3National Center HIV/AIDS, Viral
Hepatitis, STD, & TB,
Centers for Disease
Control and Prevention,
Atlanta, GA
Objective: Review
the actual and
theoretical evidence for
transmission of
bloodborne pathogens
between consecutive
vaccines by
multi-use-nozzle jet
injectors (MUNJIs) and
bifurcated needles (BNs)
without intervening
sterilization.
Background: After
smallpox eradication,
evidence emerged that
its vaccine-delivery
devices could transmit
bloodborne pathogens
such as hepatitis B (HBV)
through inherent design
defect or unsafe use.
Learning from past
mistakes might prevent
future ones with novel
delivery methods. Methods: Published literature and unpublished CDC data on high-speed, multi-use-nozzle jet injectors (MUNJIs) and bifurcated needles (BNs) were reviewed, along with standards for auto-disabling needle-syringes and related policies on vaccine safety and nosocomial disease. Smallpox eradication experience of authors and others was recalled, and novel vaccination technologies assessed. Results: Laboratory and animal model studies, epidemiologic investigations and analyses, and human trials of the Ped-O-JetR and similar MUNJIs once used in smallpox campaigns demonstrated these “jet guns” capable of HBV transmission, even when nozzles were alcohol-swabbed between injections per manufacturer instructions. In the 1990s, the Ped-O-JetR was recalled, its use abandoned by the U.S. military, and contraindicated by WHO and CDC. The latest 2008 study detected HBV contamination by PCR after 8% of MUNJI injections of HBV-carrier volunteers. The BNs were sometimes re-used in eradication without sterilization by minimally-trained vaccinators in challenging field environments. Without health officer supervision to assure sterilization and lacking auto-disabling features, BNs would not satisfy current WHO and UNICEF policies for safe injection. Although HBV transmission during smallpox eradication cannot be documented retrospectively, many involved countries have moderate-to-high prevalence of chronic infection, suggesting transmission opportunities. Conclusion: Some iatrogenic infections with HBV likely occurred in countries where unsafe MUNJIs and unsterile BNs were used. Nonetheless, the overall benefits of eradication are overwhelmingly positive and lasting. Modern emphasis on injection safety should apply to future vaccine delivery systems now in development which re-use vaccine pathways.
References: 1. Weniger BG, Papania MJ. Alternative Vaccine Delivery Methods [Chapter 61]. In: Plotkin SA, Orenstein WA, Offit PA, eds. Vaccines, 5th ed. Philadelphia: Elsevier/Saunders; 2008;1357-1392. 2. Kelly K, Loskutov A, Zehrung D, et al. Preventing contamination between injections with multiple-use nozzle needlefree injectors: A safety trial. Vaccine. 2008;26(10):1344-1352.
|
|
|
|