|
|
||||||||||||||||||||||||||||||||
|
|
|
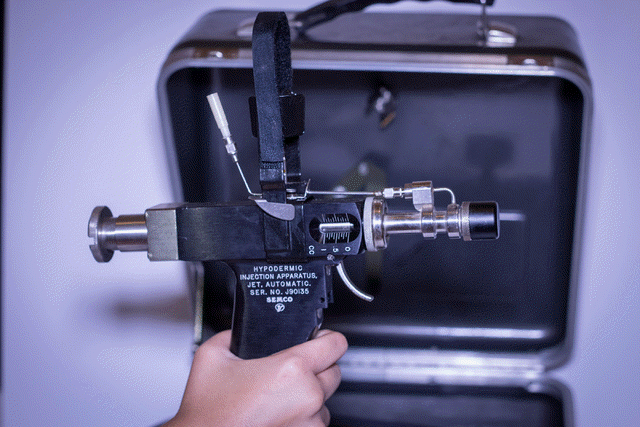
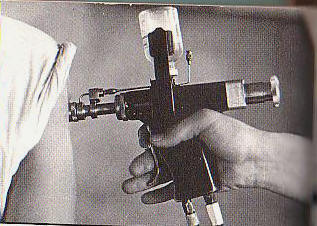
This potential risk for disease transmission would
exist if the jet injector nozzle became contaminated with blood during
an injection and was not properly cleaned and disinfected before
subsequent injections.
http://www.cdc.gov/mmwr/preview/mmwrhtml/00025027.htm CDC- MMWR Recommendations and Reports January 28, 1994 / 43(RR01);1-38General Recommendations on Immunization Recommendations of the Advisory Committee on Immunization Practices (ACIP) The following CDC staff members prepared this report:* John C. Watson, MD, MPH Charles W. LeBaron, MD Sonja S. Hutchins, MD, MPH Stephen C. Hadler, MD Walter W. Williams, MD, MPH National Immunization Program, CDC Jet Injectors Jet injectors that use the same nozzle tip to vaccinate more than one person (multiple-use nozzle jet injectors) have been used worldwide since 1952 to administer vaccines when many persons must be vaccinated with the same vaccine within a short time period. These jet injectors have been generally considered safe and effective for delivering vaccine if used properly by trained personnel; the safety and efficacy of vaccine administered by these jet injectors are considered comparable to vaccine administered by needle and syringe. The multiple-use nozzle jet injector most widely used in the United States (Ped-o-Jet) has never been implicated in transmission of bloodborne diseases. However, the report of an outbreak of hepatitis B virus (HBV) transmission following use of one type of multiple-use nozzle jet injector in a weight loss clinic and laboratory studies in which blood contamination of jet injectors has been simulated have caused concern that the use of multiple-use nozzle jet injectors may pose a potential hazard of bloodborne-disease transmission to vaccine recipients (10). This potential risk for disease transmission would exist if the jet injector nozzle became contaminated with blood during an injection and was not properly cleaned and disinfected before subsequent injections. The potential risk of bloodborne-disease transmission would be greater when vaccinating persons at increased risk for bloodborne diseases such as HBV or human immunodeficiency virus (HIV) infection because of behavioral or other risk factors (11,12). Multiple-use nozzle jet injectors can be used in certain situations in which large numbers of persons must be rapidly vaccinated with the same vaccine, the use of needles and syringes is not practical, and state and/or local health authorities judge that the public health benefit from the use of the jet injector outweighs the small potential risk of bloodborne-disease transmission. This potential risk can be minimized by training health-care workers before the vaccine campaign on the proper use of jet injectors and by changing the injector tip or removing the jet injector from use if there is evidence of contamination with blood or other body fluid. In addition, mathematical and animal models suggest that the potential risk for bloodborne-disease transmission can be substantially reduced by swabbing the stationary injector tip with alcohol or acetone after each injection. It is advisable to consult sources experienced in the use of jet injectors (e.g., state or local health departments) before beginning a vaccination program in which these injectors will be used. Manufacturer's directions for use and maintenance of the jet injector devices should be followed closely. Newer models of jet injectors that employ single-use disposable nozzle tips should not pose a potential risk of bloodborne disease transmission if used appropriately.
UPDATE: Jetgun- New PCNIF Fail Safety Test -The study ended early because the protector cap needle-free injector (PCNFI) failed to prevent contamination in the first batch tested (8.2% failure rate). Vaccine. 2008 Mar 4;26(10):1344-52. Epub 2008 Jan 18.
|
|